System Modification
The system modification track of Zurich Heart investigates new technologies that are required to improve the performance of existing ventricular assist devices (VADs). Based on clinical experience, a number of key weaknesses inherent to contemporary VADs were identified. Their elimination based on implementation of superior new technology will significantly advance handling and performance of future VADs. Patients will benefit from improved life expectancy and comfort.
CONTINUOUS MONITORING AND ADAPTIVE OPERATION
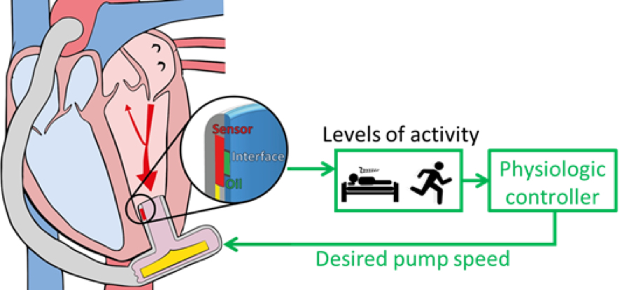
Today, ventricular assist devices typically do not adapt to the physiological requirements of the patient. Control systems to adjust the pump’s level of support according to the patient’s need are highly desirable, as they would increase the patient’s quality of life and prevent complications such as left ventricular suction.
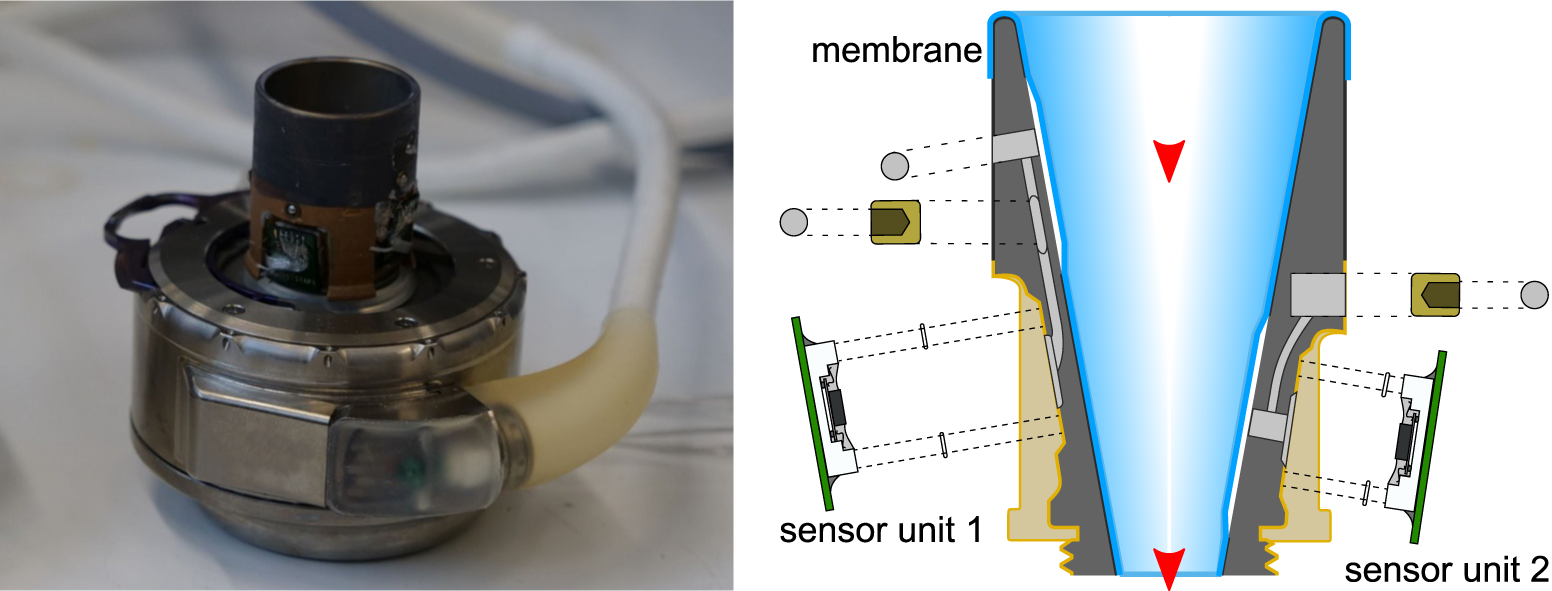
Sensors for Hemodynamic Monitoring
Prof. Christofer Hierold (ETH), Prof. Mirko Meboldt (ETH), Dr. Marianne Schmid Daners (ETH)
The development of pump-integrated or implantable sensors provides the basis for continuous patient monitoring and dynamic adaptation of pump performance to patient need. Therefore, we place a strong focus on the development of several innovative sensor concepts for the measurement of hemodynamic parameters. Our research activity has resulted in a patent portfolio covering the design and manufacturing processes for the integration of sensors for the measurement of ventricular pressure, ventricular volume and pump flow. Furthermore, we investigate sensory approaches for the direct measurement of the aortic strain, the aortic pressure, the pulse wave velocity, and the total cardiac output.
Control Systems
Prof. Mirko Meboldt (ETH), Dr. Marianne Schmid Daners (ETH)
Another essential component is the development of the algorithms that leverage the spectrum of available sensor inputs to ensure both early warning and prevention of complications. The aim is to meet a variety of objectives such as physiological pump flow adaptation to varying perfusion requirements, aortic valve opening for a predefined time, augmentation of the aortic pulse pressure and prevention of left ventricular suction and over pumping. Our patented control technologies include physiological controllers that are based on left ventricular pressure features and left ventricular volume measured by ultrasound or electrocardiography. In the development of such systems, we have built extensive competencies in the in-vitro evaluation on test benches designed specifically for the respective application as well as in vivo trials.
Physiological Control and Testing
IMPROVED IMPLANTABILITY
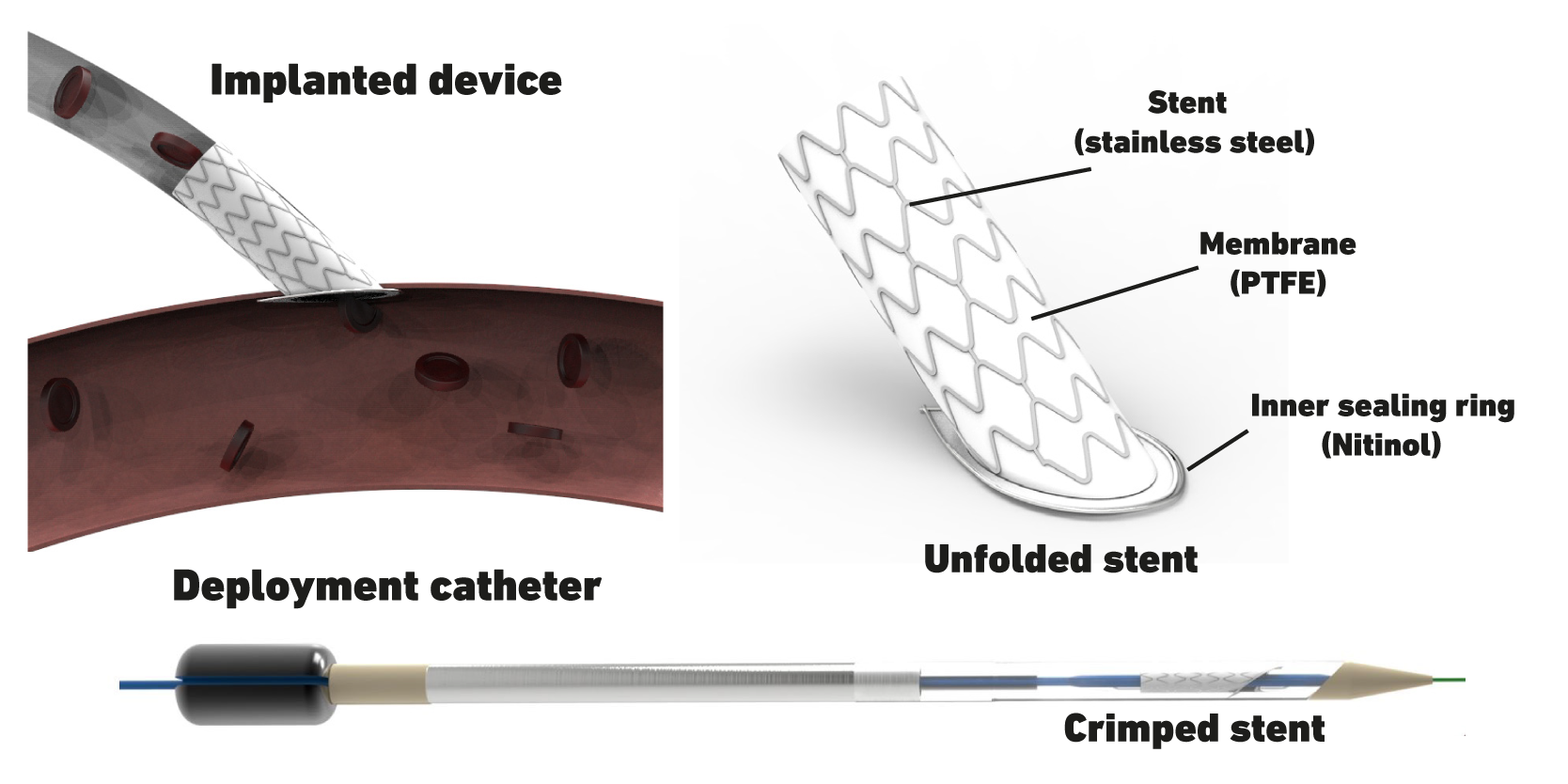
Simplified Anastomosis
Prof. Mirko Meboldt (ETH), Dr. Marianne Schmid Daners (ETH)
We not only work towards optimizing VADs, but also towards developing technologies for their implantation that are less invasive. In particular, we focus on the connection of the VAD’s outflow graft to the aorta – the so-called anastomosis. A novel catheter-based, minimally invasive delivery device was developed that deploys an acute-angled stent inside the aortic wall, ensuring sealing of the pressurized system during the entire implant procedure. This procedure can replace manual suturing and thus can be four times faster than hand sewn anastomosis. Currently, we work towards developing our prototype to a reliable and safe system and further demonstrate safety and efficacy. Additionally, we explore the application of the developed device in other end-to-side vascular connections beyond VAD implantation.
CAPABILITIES FOR VAD DEVELOPMENT
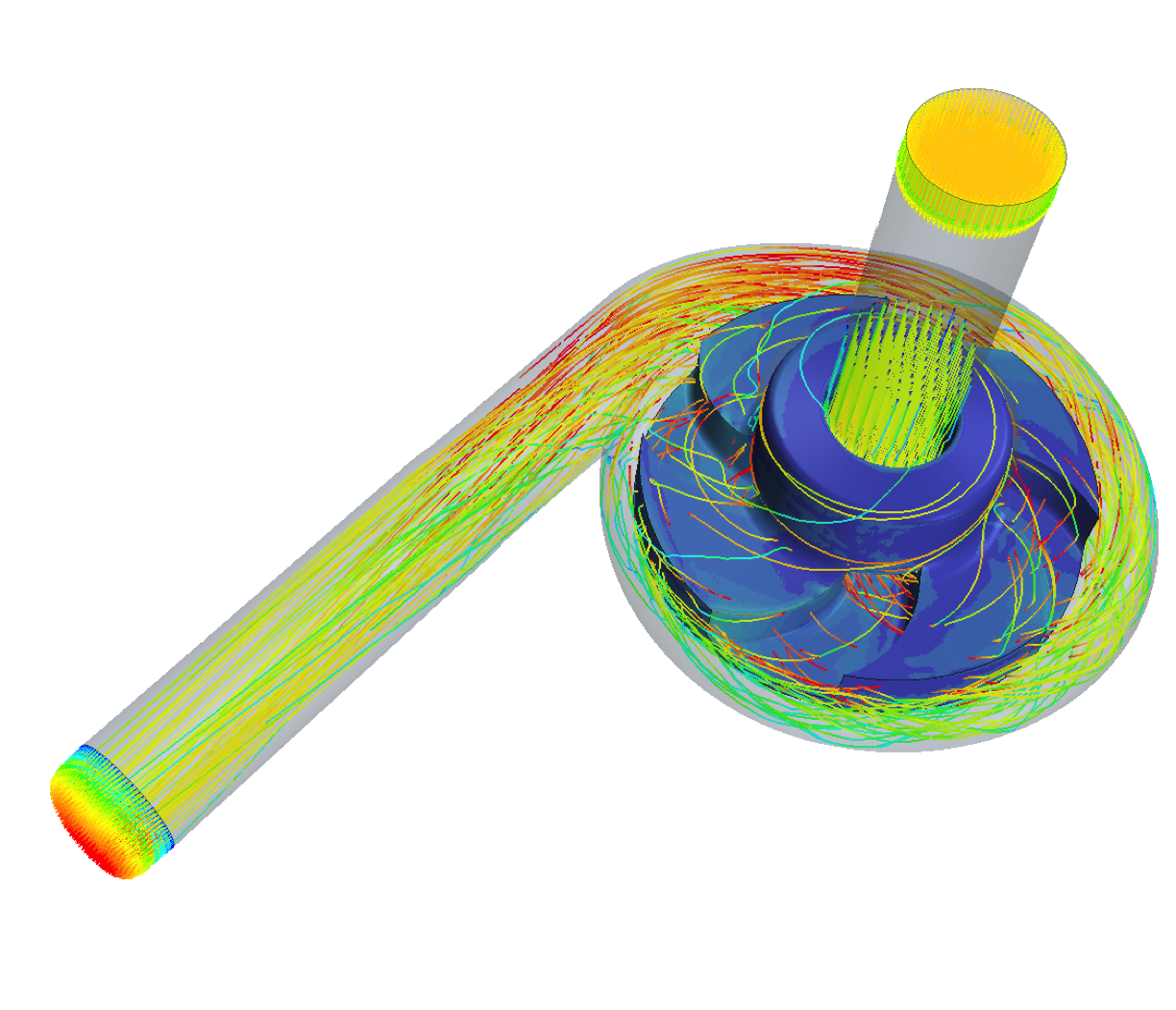
Another important aspect is the continuous improvement of flow-related hemocompatibility and pump efficiency by hydraulic design of impeller and housing of future pumps. The aim is to achieve the required hydraulic performance to support the human circulation while at the same time keeping the hemocompatibility at a tolerable level. As a research consortium, we are active in the entire design and validation chain from in silico simulations to in vivo experiments and are constantly advancing the methods and processes. In the past, this has allowed us to contribute to the development of design criteria for hemocompatibility and also to apply our findings in collaboration with industry to optimize existing pumps.
Haemodynamic Optimization
Prof. Vartan Kurtcuoglu (UZH)
The biophysical interaction between ventricular assist devices (VAD) and blood is critically influenced by fluid dynamics: Areas of stagnating flow harbour the risk of increased thrombogenesis, while regions of high shear stress can lead to blood damage. Furthermore, the non-pulsatile nature of current devices exposes the entire arterial vasculature to non-physiological flow and while the underlying mechanisms are not yet understood evidence is accumulating that the lack of pulsatility could have detrimental implications for the vasculature’s health.
The primary aim of this project is to optimize haemodynamics in the VAD for the reduction of haemolysis, thrombosis and bleeding. To this end, we have developed a computational framework to probe the local hemodynamics in VADs under operating conditions. At the same time, we are conducting in vitro studies to investigate the impact of the VAD’s non-pulsatile blood propulsion on the secretion of von Willebrand Factor, an important protein in haemostasis. Ultimately, such experimental data will widen the scope of haemocompatible blood propulsion from a device-focused to a system-wide view and could introduce pulsatility as necessary requirement for future VADs.
In vitro Testing with Mock Circulations
Prof. Mirko Meboldt (ETH), Dr. Marianne Schmid Daners (ETH)
We operate advanced mock circulations for the evaluation of ventricular assist devices, biventricular assist or total artificial hearts, which are based on a hardware-in-the-loop concept. A numerical model of the human blood circulation runs in real-time and computes instantaneous pressure, volume, and flow rate values. The device to be tested is connected to a numerical-hydraulic interface, which allows the interaction between the device and the numerical model of the circulation and thus the evaluation of the performance of the device. We also implemented viscosity control that allows to accurately mimic viscosity changes of blood as they could occur in patients, for example, due to an infusion of saline solution or anemia.
Hybrid Mock Circulation for LVADs
Hybrid Mock Circulation for BiVAD and TAH Support or Fontan Circulation
Ex vivo Testing of Blood Damage
To determine the blood damage caused by different pump designs, we have set up a blood laboratory where multiple pumps can be efficiently tested in parallel. Thanks to access to blood testing capacities of the university hospital, we have been able to build an understanding of how to further improve the standard test protocols currently in use. In addition, the proximity to the local slaughterhouse provides us with a unique supply of animal blood for testing.
Iterative Development with Additive Manufacturing
Prof. Mirko Meboldt (ETH), Dr. Marianne Schmid Daners (ETH)
We benefit from the extensive research on additive manufacturing at ETH Zurich. This has given us a holistic understanding of the benefits of additive manufacturing for product and process improvements, which we can also apply to the further development of ventricular assist devices. We have manufactured pump parts from plastics, titanium, and even magnetic composites, taking advantage of exceptional design freedom as well as rapid prototyping. In doing so, we are establishing criteria for the applicability of additive manufactured parts for use in various test phases and integration stages.
In vivo Validation
The expertise and infrastructure needed for animal trials is available.
NEW MATERIALS
Low-thrombogenic metallic glasses
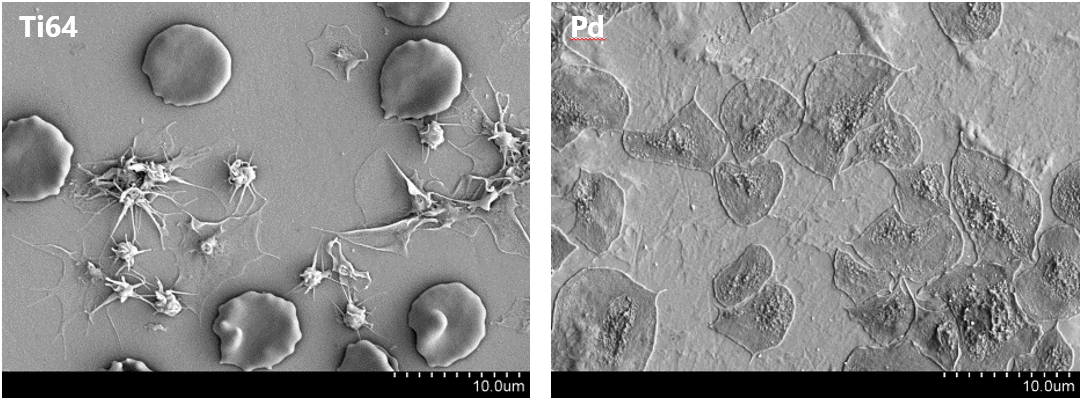
Prof. Jörg F. Löffler (ETH), Prof. Katharina Maniura (Empa/ETH), Dr. Markus Rottmar (Empa)
While metals have become a standard material class for blood-contacting devices, they usually require complex surface coatings and systemic anticoagulation therapy. Metallic glasses are interesting materials for blood-contacting devices due to their increased strength and elasticity, as well as their superior corrosion and wear resistance compared to their crystalline counterparts.
Our goal is to better understand the impact of material surface physiochemical properties and material bulk atomic order on protein adsorption, platelet activation and subsequent blood coagulation as well as endothelial cell attachment to metallic glass surfaces. An in-depth understanding of how blood and cells interact with metallic glasses will allow us to develop new materials that provide a long-term anti-thrombogenic properties.
ENERGY SUPPLY AND COMMUNICATION
Transcutaneous Energy and Information Transfer (completed project)
Prof. Johann W. Kolar (ETH)
One of the main challenges with the use of current VADs is the energy supply. Implanted devices are powered by an external energy source via a driveline penetrating the skin. To reduce the associated risk of infection and to improve patients’ comfort, wireless transcutaneous energy transfer and storage is needed. The main goal of this subproject is to develop a closed loop controlled VAD that is linked wirelessly to an external control unit to transfer energy and exchange information.